Your questions answered
To submit a question to our Agronomists please click here.

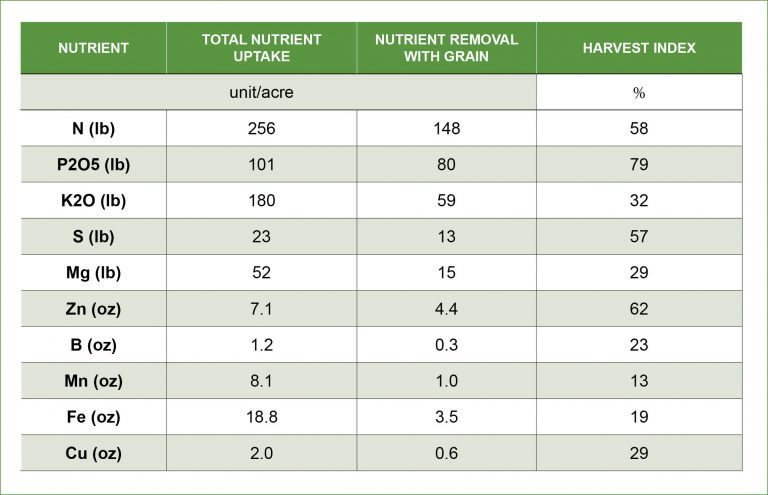
Q: What is the difference between nutrient uptake and nutrient removal? How are they used to calculate nutrient harvest index?
A: When developing a nutrient management plan, there are two essential concepts to utilize: nutrient uptake and nutrient removal. Nutrient uptake is the amount of each nutrient required for the crop to complete its life cycle at a given yield level. Nutrient removal is calculated by taking the concentration of each nutrient in harvested material and multiplying by the harvest yield. Generally, uptake levels are higher than removal of nutrients in grain crops like corn (table below). However, in crops like alfalfa or silage where the majority of the above ground biomass is being removed from a field, removal levels can be approximately equivalent to uptake. These values are essential for determining nutrient application rates for current and future crops.
Nutrient harvest index is calculated by dividing the nutrient removed by the nutrient uptake then multiplied by 100 to get a percent. In this example, corn has a high phosphorus harvest index, which means that approximately 79 percent of the phosphorus that is taken up is removed in the grain. Harvest index is especially important when considering nutrient management decisions. Since a high portion of the phosphorus is translocated to the grain and removed, it is important to make sure yields are not phosphorus limited and adequate levels are supplied for subsequent crops.

Q: I’ve heard a lot of discussion about 2X2X2 placement of fertilizer in bands. Is there anything I should know if I try this approach?
A: Banding is a good nutrient application method for two reasons: usually it means that fertilizer applications are made below the soil surface (not always the case though – surface banding of nitrogen as an example) decreasing the potential for loss via surface transport, and creating nutrient rich bands can slightly increase availability of the nutrient to the crop (not as much as is often reported).